“The important thing is not to stop questioning. Curiosity has its own reason for existence.” — Albert Einstein
Alright, let’s talk about something super simple: light hits metal, and BAM! Electrons just fly out of it. This was the weird reality physicists were wrestling with in the early 1900s, and no one really knew why it was happening. They had light, they had metal, and they had some seriously confused scientists. Enter: Einstein. Let’s rewind.
The Problem No One Could Solve
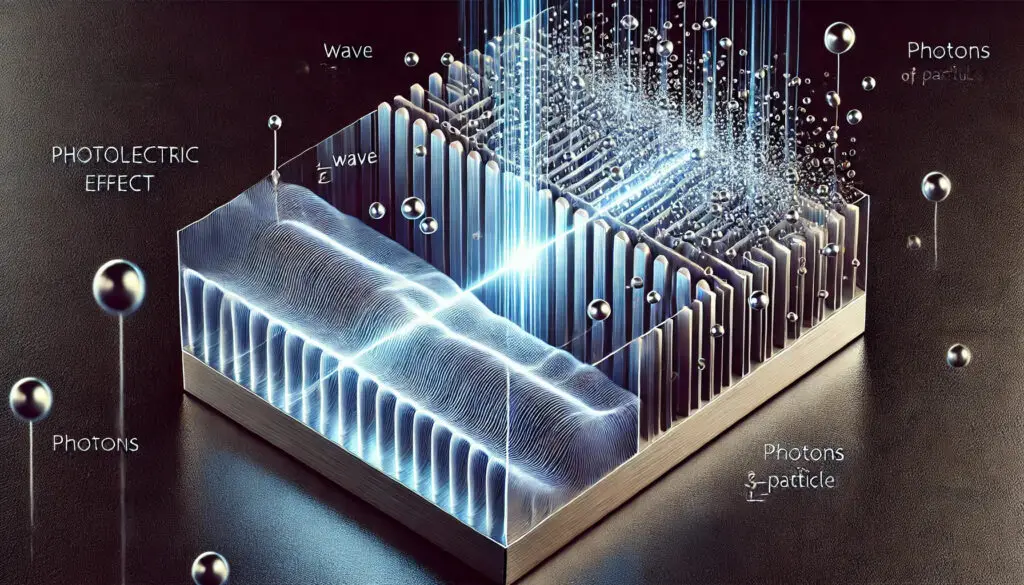
Imagine you’re a scientist, and you’ve discovered this strange thing: if you shine light on metal, sometimes electrons pop off the surface like they’ve been slapped by an invisible hand. Weird, right? You think, “Okay, light’s a wave (everyone knows that), so the brighter the light, the more energy it should have. More energy = more electrons flying off the metal. Easy.”
Except, it doesn’t work like that.
You could shine a super dim blue light, and electrons would still jump off like they’d had too much coffee. But shine the brightest red light, and — nothing. Crickets. The electrons won’t budge. What on Earth was going on?
This wasn’t a one-time thing. It happened over and over, with different metals, different lights, and always the same confusing result: only certain colors of light (high-frequency ones, like blue or ultraviolet) could knock electrons loose. No matter how intense the light was, if it was red or any other low-frequency light, those electrons stayed stubbornly put.
Enter Einstein: The Guy Who Changed Everything
Here’s where Einstein strolls in with a huge grin and a “what if” that changed the course of physics forever. “Guys,” he says (and I’m paraphrasing here), “What if we’re thinking about light all wrong? What if light isn’t just a wave? What if it’s also… a particle?”
This was a time when people were still wrapping their heads around the whole “light as a wave” thing. And now Einstein’s saying, “Nah, light’s got another side to it — it’s like, part-wave, part-particle.” Specifically, he proposed that light is made up of these tiny little packets of energy called photons. Each photon carries a specific amount of energy, and that energy depends on the frequency of the light. High-frequency light (blue, UV) = more energetic photons. Low-frequency light (red) = chill, low-energy photons.
But here’s the kicker: these photons weren’t just rolling up to the metal and being all “hello, electrons, care to move?” They were straight-up kicking electrons out, like an atomic game of dodgeball. But they needed enough energy to do it. And here’s where Einstein’s genius comes in:
No matter how many photons you throw (aka how bright the light is), if those photons don’t have enough energy, they’re not kicking out any electrons.
The “Aha!” Moment
Einstein’s idea was like one of those moments in movies where the detective solves the case. The photoelectric effect finally made sense. The reason why red light didn’t knock electrons out wasn’t because there weren’t enough photons. It was because each photon was too weak. It’s like trying to knock over a wall with cotton balls — it doesn’t matter how many you throw, it’s not happening. But blue light? Those photons had enough energy to get the job done. Think of them as the Hulk of photons.
Suddenly, the whole “frequency matters more than intensity” thing made sense. Einstein had cracked the code. He figured out that if light’s frequency (color) is high enough, the photons can pack enough punch to knock electrons out of the metal. If not? Those electrons are staying cozy.
Why Should You Care?
Now, you might be thinking, “Cool story, but why does this matter?” Oh, let me tell you why. This idea — that light is made of photons — was the birth of quantum physics. Yeah, that’s right, the same quantum physics that gave us things like computers, lasers, and every mind-bending sci-fi concept you can think of.
And here’s the wild part: even though most people associate Einstein with relativity (because, you know, E=mc²), he won the Nobel Prize for explaining this weird electron-popping effect. This was the big deal. This was the start of thinking about the universe in a completely different way.
Oh, and also? Solar panels? They work because of this exact thing. Every time sunlight hits a solar panel and generates electricity, the photoelectric effect is at work. Einstein’s photons are punching those electrons right off the surface and into your phone battery.
Wrapping It Up
So, the next time you’re sitting in the sun, soaking up those rays, think about Einstein. Think about how a simple question — “Why do electrons only jump off with certain colors of light?” — led to one of the biggest scientific revolutions ever. Einstein’s genius wasn’t just in figuring out what was going on; it was in having the imagination to say, “What if light isn’t what we think it is?”
And that’s how a curious guy with a question about electrons gave us the photon, and in doing so, changed physics forever.